
NUTRICIA NORTH AMERICA, INC. (“NUTRICIA”) BRAND PROTECTION POLICY
Effective Date: November 22, 2021 This Brand Protection Policy (the “Policy”) is issued by Nutricia and applies to Resellers of Nutricia products (“Products”), in the United States of America, and supplements any then-current policy or agreement between Reseller and Nutricia, including Nutricia’s effective General Terms and Conditions of Sale (T&Cs). By purchasing Products from Nutricia or a Nutricia Authorized Distributor, Reseller agrees to adhere to these additional terms and conditions. Until such status is revoked by Nutricia, Reseller shall be considered an Authorized Reseller. Nutricia may review Reseller’s activities for compliance with this Brand Protection Policy. 1. Authorized Customers. Reseller is only authorized to sell Products to End User Consumers. An “End User Consumer” is a purchaser of the Products who is the ultimate consumer of the Products and who does not intend to resell the Products to any third party. Reseller shall not sell or transfer Products to any person or entity Reseller knows or has reason to know intends to resell the Products. Reseller shall not sell or transfer a quantity of the Products to any End User Consumer greater than that typically purchased for personal use. 2. No Online Sales. Reseller shall not sell Products on or through any website, online marketplace (including but not limited to Amazon and eBay), mobile application, or other online forum without Nutricia’s prior written consent. To apply for such consent, Reseller shall submit an Authorized Online Reseller Application and if approved, consent will be granted through the “Letter of Limited Authorization to Sell Nutricia Products Online”. The terms of this Policy supersede any prior agreement between Nutricia and Reseller regarding the sale of the Products on or through websites, mobile applications, and other online forums, and any authorization previously granted to Reseller to sell online is revoked. 3. Sales Practices and Quality Controls. Reseller shall not engage in any deceptive, misleading, or unethical practices and shall not make any warranties or representations concerning the Products except as authorized by Nutricia. Reseller shall comply with all instructions provided by Nutricia regarding the storage, handling, shipping, disposal, or other aspect of the Products, including instructions provided on Product labels and the Product Quality Guidelines attached as Exhibit A, as may be amended by Nutricia. 4. Intellectual Property. Reseller agrees that Nutricia or its licensors own the Nutricia IP, as defined in Nutricia’s T&Cs. Reseller is granted a limited, non-exclusive, non-transferable, revocable license to use the IP solely for marketing purposes and selling the Products as set forth herein. This license will cease upon termination of Reseller’s status as an Authorized Reseller. Nutricia reserves the right to review and approve Reseller’s use of the Nutricia IP at any time. 5. Termination and Modification. If Reseller violates this Policy, Nutricia reserves the right to terminate Reseller’s status as an Authorized Reseller upon written notice, via email or other electronic means. Nutricia reserves the right to update, amend, or modify this Policy at any time. Unless otherwise provided, such amendments will take effect immediately and Reseller’s continued use, advertising, offering for sale, or sale of the Products, use of the Nutricia IP, or use of any other information or materials provided by Nutricia to Reseller will be deemed Reseller’s acceptance of the amendments. EXHIBIT A
PRODUCT QUALITY GUIDELINES 1. Site Security 1.1. There must be clear and proper boundaries for the warehouse compound to deter intruders. 1.2. All fencings must be well secured. 1.3. The Products must be stored internally under all circumstances and situations and should never be stored externally even if temporarily. The Products must be stored inside the warehouse in secure and dry conditions. 1.4. There should be security checks on all movement of vehicles and staff within the warehouse site, with clear documented processes. Security personnel must be well trained to meet security requirements. 2. Housekeeping of External Areas 2.1. The warehouse should have areas designated for waste segregation, and that there is proper isolation of common waste and pallets. 2.2. The warehouse site must always be clear of any rubbish, debris, obsolete or surplus materials. 2.3. All waste receptacles should be leak-tight with tight-fitting lids or covers. 2.4. Hazardous materials, liquids or liquid-containing wastes should never be placed in a dumpster or trash receptacle. 3. Hardstanding Areas and Roadways 3.1. The warehouse must have adequate drainage to ensure 100% dryness of the warehouse under all conditions. There should not be water pools, mud or any source of dampness inside or outside the warehouse, even during rainy seasons. 4. Buildings and Other Structures 4.1. The maximum protection of the Products should be guaranteed at all times through the proper maintenance, design and construction of the warehouse. 4.2. Building design and construction materials should be suitable to adequately protect goods stored inside from all potential threats including weather conditions, dust or dirt particles, pests and other damages. 4.3. Fuels or any other liquid contaminants are not allowed for storage within the warehouse premises to avoid any risk of contamination or food safety risks. 4.4. All battery charging areas and sources of fuels should be clearly separated from the products and personnel to avoid any food safety or personnel safety risks. 5. Internal Areas 5.1. Glass and other breakable materials, e.g. hard plastics, have to be kept to a minimum and should not pose a risk. 5.2. All breakages should be checked on a regular basis and reported immediately to ensure that no glass fragments are scattered on the products. All windows, plastic lamps, equipment glass, light bulbs, any picture frames and other breakable materials should be preferably protected with a shatterproof film or casing or should be made of appropriate materials. 5.3. There must be a good ventilation system in the warehouse. 6. Temperature Conditions: 6.1. The temperature at the warehouse premises and storage areas must be adequately controlled at a daily temperature range of + 5°C to + 40°C to avoid any food safety risks. 6.2. Liquid milks must be stored within a daily temperature range of +5°C to +25°C to avoid any food safety risks. 6.3. Ready to Feed’s must be stored in temperature controlled warehouse. 6.4. The above temperature range must be maintained and measured both at ground and ceiling level. 6.5. During audits, Nutricia may request the temperature data logger to check the minimum and maximum temperatures that the products are exposed to per month. 6.6. The temperature storage conditions for the Products are following: 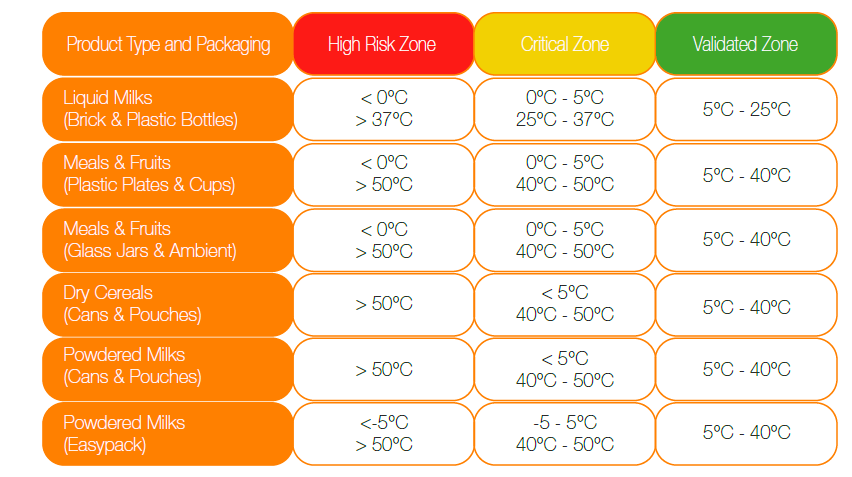
The temperatures in High Risk Zone and Critical Zone columns above are maximum daily temperatures. 6.7. The Products must be stored out of the line of direct sunlight. 6.8. The warehouse building must always be dry and sufficiently protected from humidity and condensation as any risk of moisture will affect our products or their external packaging. 7. Buildings and Storage Areas 7.1. The racking space must comply with quality and safety rules to ensure that there is NO risk to personnel working under the storage racks. 7.2. There must be clear visual and written instructions visible across the warehouse on the approved stacking configuration of the Products on or off pallets, with or without storage racking i.e. the permissible maximum number of cartons high. The warehouse site must comply strictly with these instructions. 7.3. For all milk powder pallets, the maximum pallet height is 2.50m. 7.4. It is necessary to maintain adequate spacing between storage racking or pallets and walls and structures to allow for periodic cleaning, to prevent dampness and damage to the products and overcrowding of the stored goods. 7.5. All products must be stored on pallets and on the racking at a minimum of 50cm from the wall. 7.6. The racking should be accessible from both ends. 7.7. Pallet must not lean against wall or skid on packaging material. 8. Welfare Facilities 8.1. There must be adequate sanitary facilities and wash basins within the warehouse for all warehouse personnel in addition to specific and documented procedures to ensure their constant cleanliness. 8.2. Good hygienic practices must be observed across all the facilities and activities within the warehouse especially when warehouse employees are handling the Products including picking and returns. 9. Equipment 9.1. All the equipment used within the warehouse must be fit for their intended purpose and fit for use to avoid any food safety or personnel safety risks. 9.2. There must be contingency plans and back up plans in place to prevent breakdowns and disruption. 10. Non-Conforming Products and Returns 10.1. Non-conforming Products must be segregated both physically and in the electronic systems for sorting and inspection. These Products must be stored separately in an inspection area. 10.2. All damaged Products should be separated for disposal. This includes wet, torn, open or expired Products. 10.3. All Products in the original carton should be kept aside. 10.4. Batch numbers and Best before Date must be checked and complied with. 10.5. There must be a clear and proper documentation of non-conforming Products arising from returns, co-packing and damages across all stages of the Distributor process. 10.6. There must be a clear and documented procedure to ensure that the condition of any non-conforming and returned Products is checked upon arrival at the warehouse. 10.7. Once the product condition of these Products is confirmed, they must be inventoried, labeled and stored correctly in a designated area. 10.8. Periodic inventory counts have to be regularly carried out to ensure accurate and updated inventory quantity figures. If discrepancies are noted, action must be taken to investigate them and adjust the stock figures. 10.9. Every finished product disposal process should be witnessed by Nutricia. 11. Cleaning 11.1. All internal areas, equipment and work surfaces must be kept tidy and clean at all times. 11.2. Cleaning records must be updated regularly and has to be available during audit. 12. Storage of Risk Substances 12.1. Fuels cannot be stored onsite and all lubricants should be kept offsite. 12.2. Only food grade lubricants should be used for Material Handling Equipment and other equipment in the warehouse. 12.3. All identified potentially hazardous materials (Hazmats), allergens or substances must be stored in a separate and secure location and should be accessible only to authorized personnel. 12.4. All other products stored at or nearby the Products should not pose a risk of contamination or food safety in the event of leaks or damages. 13. Pest Control 13.1. It is mandatory to have a formalized pest control program in place across the warehouse focusing on preventative measures with visible traps, frequency of checks, a clear instruction with records in place. 13.2. Toxic pest baits must strictly be avoided. 13.3. The pest control company must be fully certified and has to regularly conduct inspections and replenish the bait stations. 14. Waste Disposal 14.1. All products must be properly and completely disposed without any possibility for re-use. 14.2. All packaging materials must be destroyed to prevent re-use. 14.3. Every finished Product disposal process should be witnessed by Nutricia. 15. Vehicle Unloading and Loading 15.1. All Products and materials arriving at the warehouse must be checked for the following before being received physically and entered into the Warehouse Management System: 15.1.1. Container seal integrity 15.1.2. Quantity 15.1.3. Batch number / Best Before Date 15.1.4. Overall quality of the Products The above point has to be clearly documented and followed. 15.2. All vehicles used for transportation, whether rented or own, inbound and outbound, have to be checked against a checklist for compliance to formal quality conditions of cleanliness. 15.3. Measures should be in place to prevent unauthorized persons from entering and/or tampering with vehicles and/or equipment, as well as prevent any theft. 15.4. Vehicles and containers should be of sufficient capacity to allow orderly storage of the various categories of products during transportation. 15.5. Vehicles and equipment used to distribute products to stores and retailers should be appropriately protective of the products to prevent exposure to conditions that could affect their stability and packaging integrity, as well as prevent contamination of any kind. 15.6. The Products should not be carried or distributed with other products such as fuel, fresh foods (meat, fish, vegetables, fruits, etc.), animal feed, any food that can present a threatening defect as infestation, leaks, dirt etc., chemical products and products with strong odors 15.7. Before loading the cartons in the transportation vehicle, the warehouse in-charge or the assigned staff must check the vehicle and container for leaks, rust, overall cleanliness, bad odor, rodents, dust, the temperature of the vehicle. 15.8. Deliveries should be examined at receipt in order to check that containers are not damaged and that the consignment corresponds to the order. 15.9. Where controlled conditions (for example, temperature, relative humidity, light, etc.) are required during transit, the recipient should examine the shipment upon reception to ensure the conditions have been met and record the results. 15.10. The Products should be promptly transferred to the appropriate, environmentally controlled storage area. 15.11. Warehouse in-charge must check the temperature of the container before receiving and off-loading the shipment(s). If the temperature is within the acceptable range, the products can be off loaded and taken in to the warehouse. 15.12. The warehouse in-charge must also check the status of the pallets and the number. 16. Forklifts and Other Site Vehicles 16.1. Vehicles running on fuel are not allowed inside the storage and working area. 16.2. Only 100% electrical powered Battery Forklifts must be used at warehouse site. 16.3. All battery charging areas and sources of fuels should be clearly separated from the Products and personnel to avoid any food safety or personnel safety risks. 17. Quality Management 17.1. Distributor Employees are required to use safety equipment at all times while working with the Products. It is mandatory that they always wear the appropriate Personal Protection Equipment (PPE) including: 17.1.1. Safety helmets 17.1.2. Safety goggles 17.1.3. Safety harnesses if working from height 17.1.4. Protective ear wear 17.1.5. Safety vests and shoes 17.1.6. Gloves while moving pallets 18. People Training 18.1. The Distributor shall ensure that: 18.1.1. All relevant personnel at the warehouse are regularly trained on Food safety and hygiene training. 18.1.2. All members of the warehouse teams have undertaken the Danone Nutricia ELN Quality and Food Safety training and passed the appropriate assessments. 18.1.3. New warehouse staff dealing with the Products should begin the Danone Nutricia ELN Quality and Food Safety training within one month of commencing employment with the Distributor and should successfully pass the appropriate assessments. 18.1.4. All warehouse staff involved with the products must retake Danone Nutricia ELN Quality and Food Safety training and assessment every 12 months. 18.1.5. The Crisis Management E-learning Course must be completed by a minimum of 1 employee per Distributor. 19. Quality Management 19.1. It is mandatory that the warehouse and/or the Distributor are ISO 9001 certified or compliant. 19.2. The Distributor should report immediately to Nutricia about any adverse events. 20. Crisis Management 20.1. A risk assessment has to be carried out to identify any potential crisis that the warehouse may be exposed to, their severity and likelihood of occurrence. 20.2. In the event of a crisis, and in order to protect the integrity of Products and to minimize disruption of supply chain, a formalized management plan is mandatory. 20.3. In case of any need for Products withdrawal the Distributor should immediately notify Nutricia. 21. Management of Stored Goods 21.1. The warehouse site should have a documented procedure for stock control of the freshness of the products. 21.2. The Distributor must carry out a routine check of stock count by batch numbers every 3 months. 21.3. The Distributor must ensure that there is proper stock rotation of first expiry, first out. 21.4. The Distributor should complete a cycle stock count, wherein the Products at the warehouse are counted once a year and any deviations observed must be reported to Nutricia. 21.5. Any discrepancies between physical quantity and system records must be checked and reconciled. 21.6. The quantity and batch numbers must be exactly matching each other. 21.7. FEFO policy must be practiced for the storage, picking and dispatch of any returns or repacking activity. Storage and dispatch of goods must be according to batch and best before dates. 21.8. The warehouse has to be able to control the freshness, traceability and write offs by monitoring the aging of products inside the warehouse. 21.9. An aging report must be available to highlight any products aging beyond a defined limit or for a particular customer. 22. Traceability 22.1. There must be a clear and properly documented procedure at the warehouse to track the traceability of goods that are sold or distributed to customers at batch level or Best Before Date. This should be possible both at full and partial pallet order size. 22.2. There must be a clear and proper documented procedure to track the traceability of goods within the warehouse including incoming stocks, quarantined, returns, damaged or expired stocks or goods from co packing. 22.3. All goods and pallets must be clearly marked to enable identification for traceability and identification of freshness for both good and bad stocks at individual pallet level, batch number and Best before Date. 22.4. The warehouse must be able to track which batch is subsequently sent to the next point of sale for full, mixed and partial pallets. 22.5. In order to ensure the effectiveness of traceability at the warehouse, we advise the warehouse team to use the template on the link below to track the stock inventory. 22.6. Records demonstrating ability for tracing the goods will be requested during the audits. The Distributor must be able to supply all the data needed when requested. 23. Retention Period of all Log Sheets: 23.1. All monitoring records should be kept for a minimum of the shelf-life of the product distributed plus one year. |